
MTE INTERNATIONAL – GENOMADIX
Campobasso, Italy, 10 of June 2024: MTE International (www.mte-intl.com) is proud to announce a commercial partnership with Genomadix for the distribution in Italy and other EU Countries of the Genomadix CYP2C19 Test.
1-hour CYP2C19 Genotyping can help guide DAPT to reduce the risk of recurrent stroke in minor stroke/high risk TIA.
- The Cube is the world’s first real-time PCR point-of-need platform for precision medicine testing and other applications.
- Genomadix produces molecular tests for precision medicine genotyping and environmental/water safety testing.
- It commercializes the Cube CYP2C19 Genotyping Test — the fastest, easiest FDA-cleared test for supporting dual antiplatelet therapy decisions in stroke and cardiology patients.
MTE INTERNATIONAL – BIOSENSORS INTERNATIONAL
Campobasso, Italy, 18 of December 2023: MTE International (www.mte-intl.com) is proud to announce a commercial partnership with Biosensors International for the distribution of interventional cardiology breakthrough technologies (TAVI System) in some Italian regions.
For more than 25 years, the Biosensors International Group ("Biosensors") has been designing, manufacturing, and marketing innovative medical devices used during heart surgery and intensive care treatment.
MTE INTERNATIONAL – BLUE MEDICAL
a Translumina Group company
Campobasso, Italy, 16th of August 2023: MTE International (www.mte-intl.com) is proud to announce a commercial partnership with BlueMedical, a Translumina Group Company (link) for the distribution of interventional cardiology breakthrough technologies in some Italian regions.
BlueMedical, established in 2013, manufactures balloon catheters, including drug-coated balloons (DCB), along with other speciality balloons for complex coronary interventions.
BlueMedical has developed Protégé NC and Protégé DCB catheters to overcome current DCB technologies, offering high safety and efficacy. Protégé technology avoids any drug loss during device advancement to the lesion, ensuring high drug concentration at the targeted site.
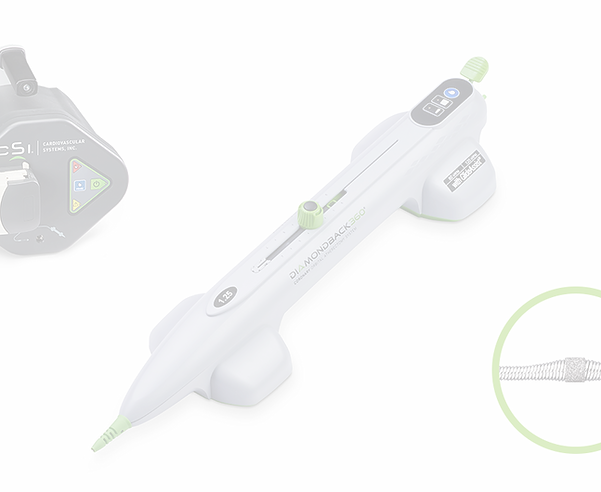
MTE INTERNATIONAL – ABBOTT MEDICAL
Campobasso, Italy, January 1st 2024: MTE International (www.mte-intl.com) is proud to announce a commercial partnership with Abbott Medical for the distribution of Diamondback 360 – Coronary Orbital Atherectomy System in the Molise region.
MTE INTERNATIONAL – MCHEALTH
Campobasso, Italy, August 2022: MTE International (www.mte-intl.com) is proud to announce a commercial partnership with McHealth Srl for the distribution of interventional cardiology breakthrough technologies in Molise Region.
MC Health is the national distributor for OrbusNeich of their range of innovative devices including an extensive portfolio of high-performance balloons (Sapphire family, Jade family), speciality catheters (Scoreflex), and microcatheters (Teleport and Teleport Control).
MTE INTERNATIONAL – MEMED
Campobasso, Italy, 1st of December 2021: MTE International (www.mte-intl.com) is proud to announce a commercial partnership with MeMed for the distribution in Italy of a breakthrough innovative technology: MeMed Covid-19 Severity™ on MeMed Key®.
MeMed COVID-19 Severity is a pioneering host-response technology that decodes signals of the immune system to accurately determine whether SARS-CoV-2 patients are likely to have a severe outcome. It does so by measuring multiple proteins from a serum sample and applying a machine-learning algorithm to stratify a patient's risk of severe outcomes.
The test runs in just 15 minutes on the company’s point-of-need platform, MeMed Key®. The test aims to help physicians identify who may benefit from escalated care and who can be safely discharged from hospital to self-isolate at home. MeMed COVID-19 Severity™ has received a CE Mark in Europe.
MTE INTERNATIONAL – TRANSLUMINA GMBH
Campobasso, Italy, 30th of April 2021: MTE International (www.mte-intl.com) is proud to announce a commercial partnership with Translumina GmbH for the distribution of interventional cardiology breakthrough technologies in Abruzzo, Marche, Molise & Umbria regions.
VIVO ISAR Stent: an innovative technology which eliminates the use of polymer by using Dual Drug Technology: Sirolimus and Probucol.
10 years follow-up clinical data of safety and efficacy in 3002 patients is published in the Journal of the American College of Cardiology. At 10-year clinical outcomes of stent thrombosis from RCTs in patients with Coronary Artery Disease, VIVO ISAR demonstrated lower definite/probable stent thrombosis rate than Xience (ISAR TEST-4) and Resolute.
Yukon Drug Eluting Stent is recommended by the latest European Society of Cardiology (ESC) guidelines for myocardial revascularization.
"Ten-Year Clinical Outcomes From a Trial of Three Limus-Eluting Stents With Different Polymer Coatings in Patients With Coronary Artery Disease – Results From the ISAR-TEST 4 Randomized Trial"
Circulation - 2018
In this unique long-term analysis at 10 years, Yukon has shown the lowest rate of definite/probable stent thrombosis with a significant risk reduction compared to Cypher (50%) and numerically lower TLR rates as compared to Xience (29%) while maintaining similar efficacy.
MTE INTERNATIONAL – DIANOSIC
Campobasso, Italy, 7th of April 2021: MTE International (www.mte-intl.com) is proud to announce a commercial partnership with Dianosic for the distribution of CAVI-T, a breakthrough innovative technology for ENT diseases, throughout the entire Italian territory.
Dianosic works with healthcare professionals to provide life-changing solutions for patients in the management of intranasal bleeding (epistaxis) and chronic sinusitis. This start-up was selected as one of the “100 Start-ups to Invest in for 2021” by Challenges.
Intranasal bleeding is very common, costly, and significantly impacts a patient’s quality of life. In chronic cases, epistaxis also affects caregivers. The incidence is rising due to aging populations and the increasing number of patients with cardiovascular diseases being treated with anticoagulants.
 English
English
